Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com

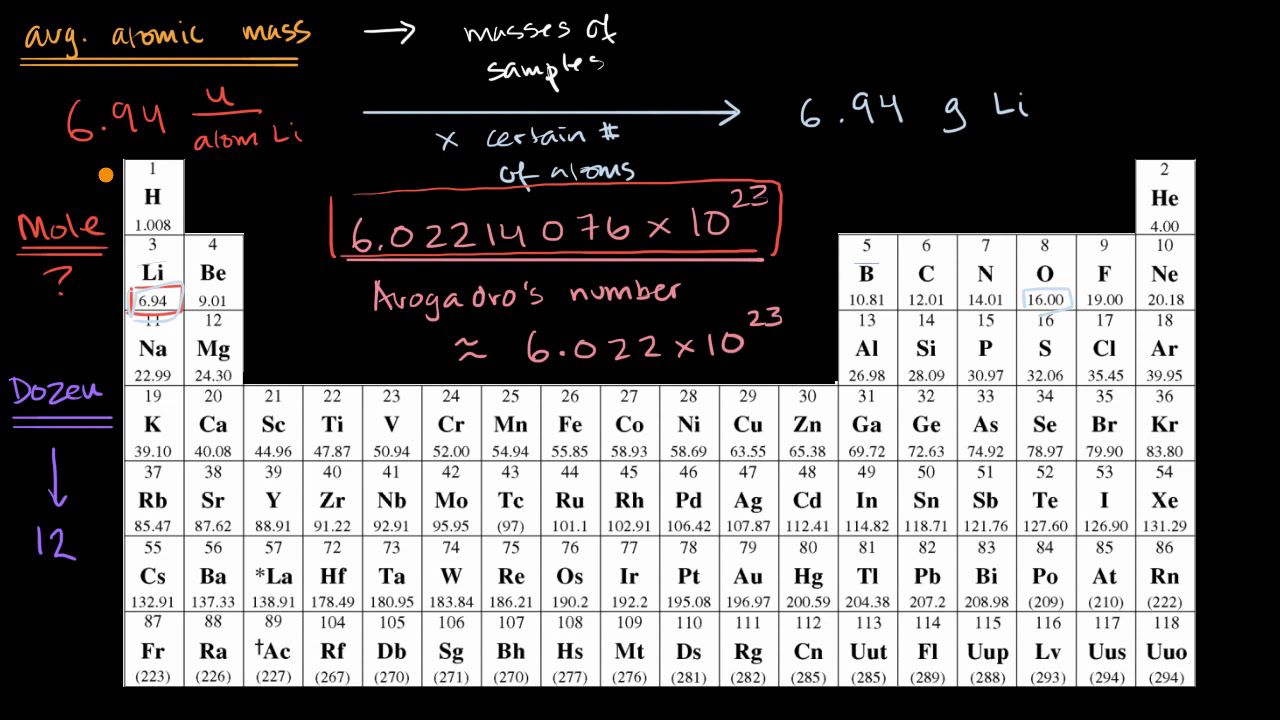
12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)

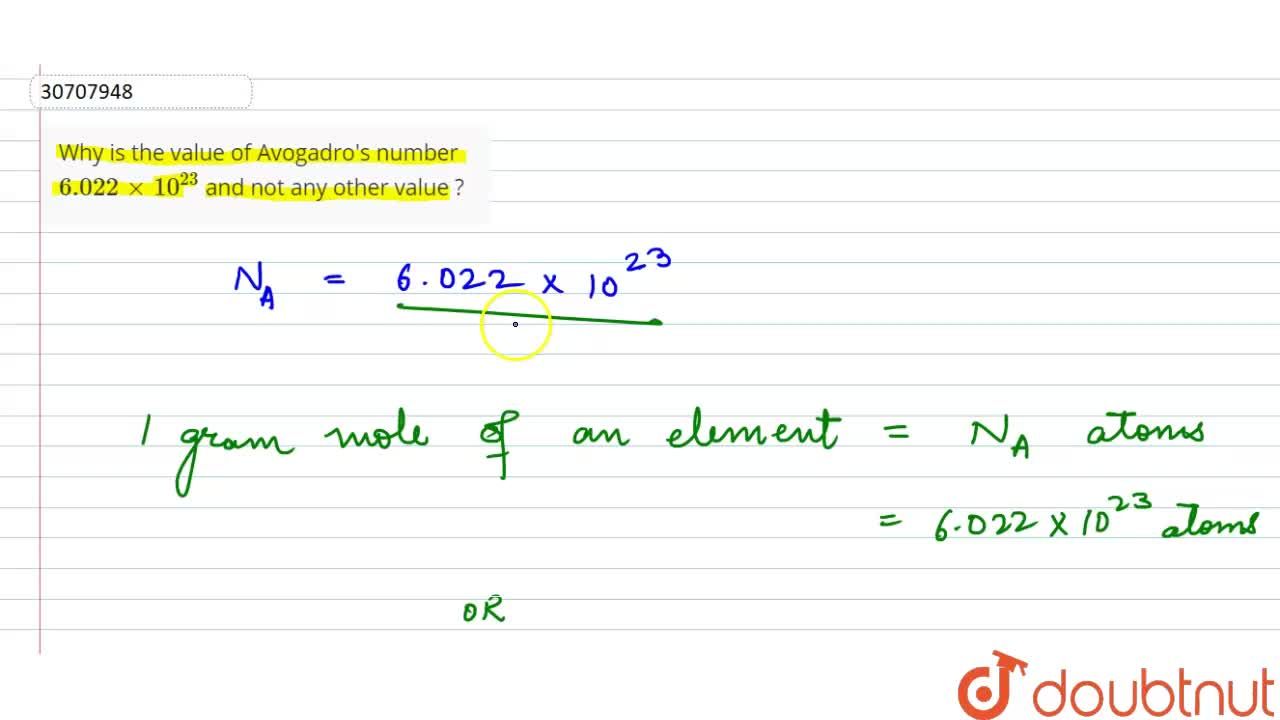
If Avogadro number NA is changed from 6.022 × 10^23 mol^-1 to 6.022 × 10^20 mol^-1 , this would change :

Atomic Mass and The Mole Topic: AMU's & Atomic Mass Objectives: Day 1 of 3 To learn how we define 1 amu (atomic mass unit) To learn how we derive atomic. - ppt download

1 mole = 6 022 x 10^23 If there is 1 mole of H2 we have multiply the Avogadro no - Science - Atoms and Molecules - 15776529 | Meritnation.com
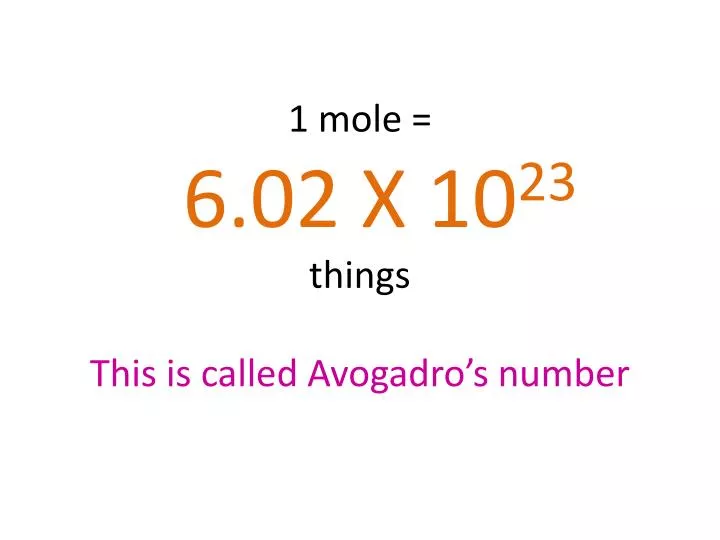
PPT - 1 mole = 6.02 X 10 23 things This is called Avogadro's number PowerPoint Presentation - ID:4272623

If Avogadro number `N_(A)` is changed from `6.022xx10^(23) mol^(-1)` to 6`.022xx10^(23) mol^(-1)`, - YouTube

Which of the following contains the same number of atoms as 4.032g of hydrogen atoms? A. 1 mole of H2 - Brainly.com