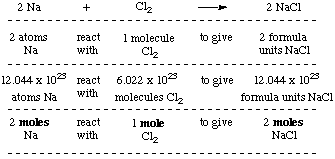The Mole What's a mole? In chemistry, a mole is a counting unit. What does that mean? –1–1 dozen = 12 units(ie eggs,donuts) –1–1 mole = 6.02x10 23 particles. - ppt download
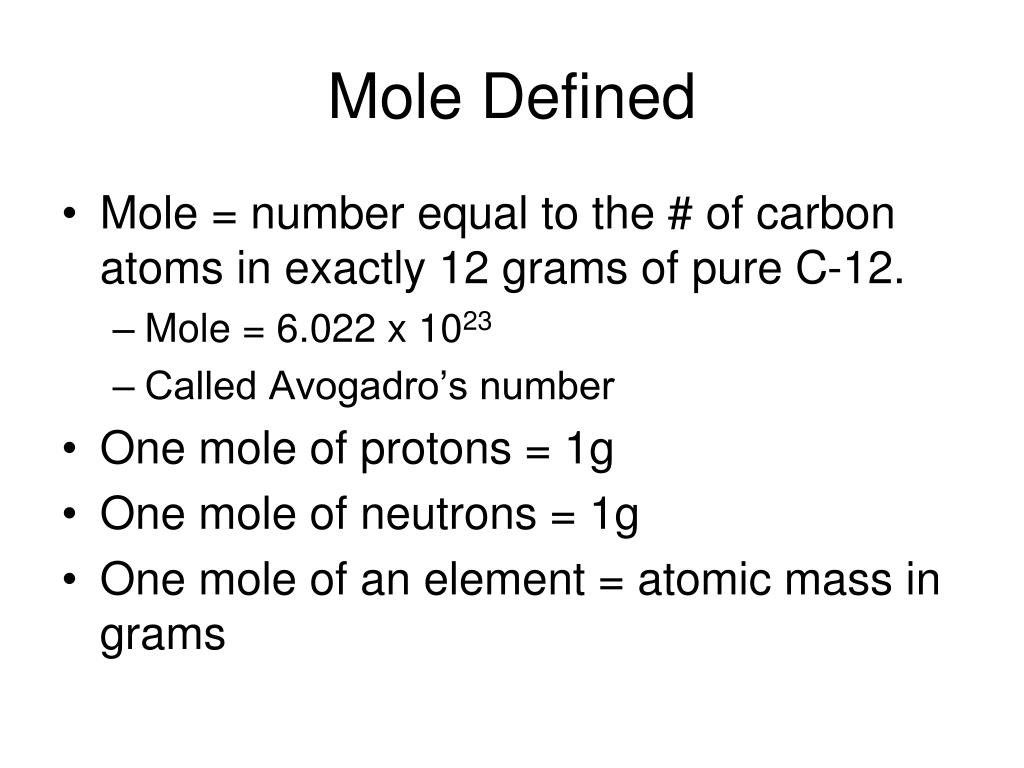
The Mole Standards 1 dozen = 1 gross = 1 ream = 1 mole = x There are exactly 12 grams of carbon-12 in one mole of carbon ppt download

Calculate the mass and charge of one mole of electrons. Given: Mass of one electron = 9.10 × 10^-31 kgCharge of one electron = 1.602 × 10^-19 coulomb

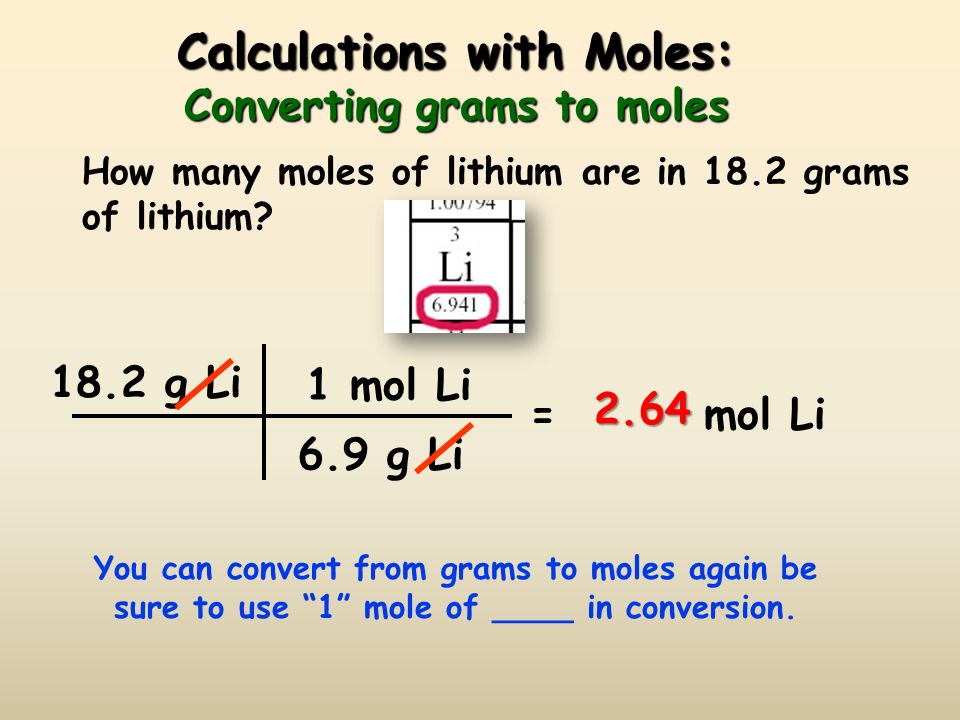
If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of 1 atom of carbon?... - YouTube

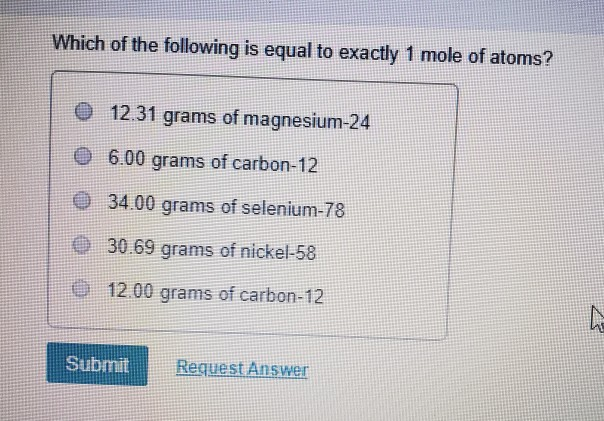


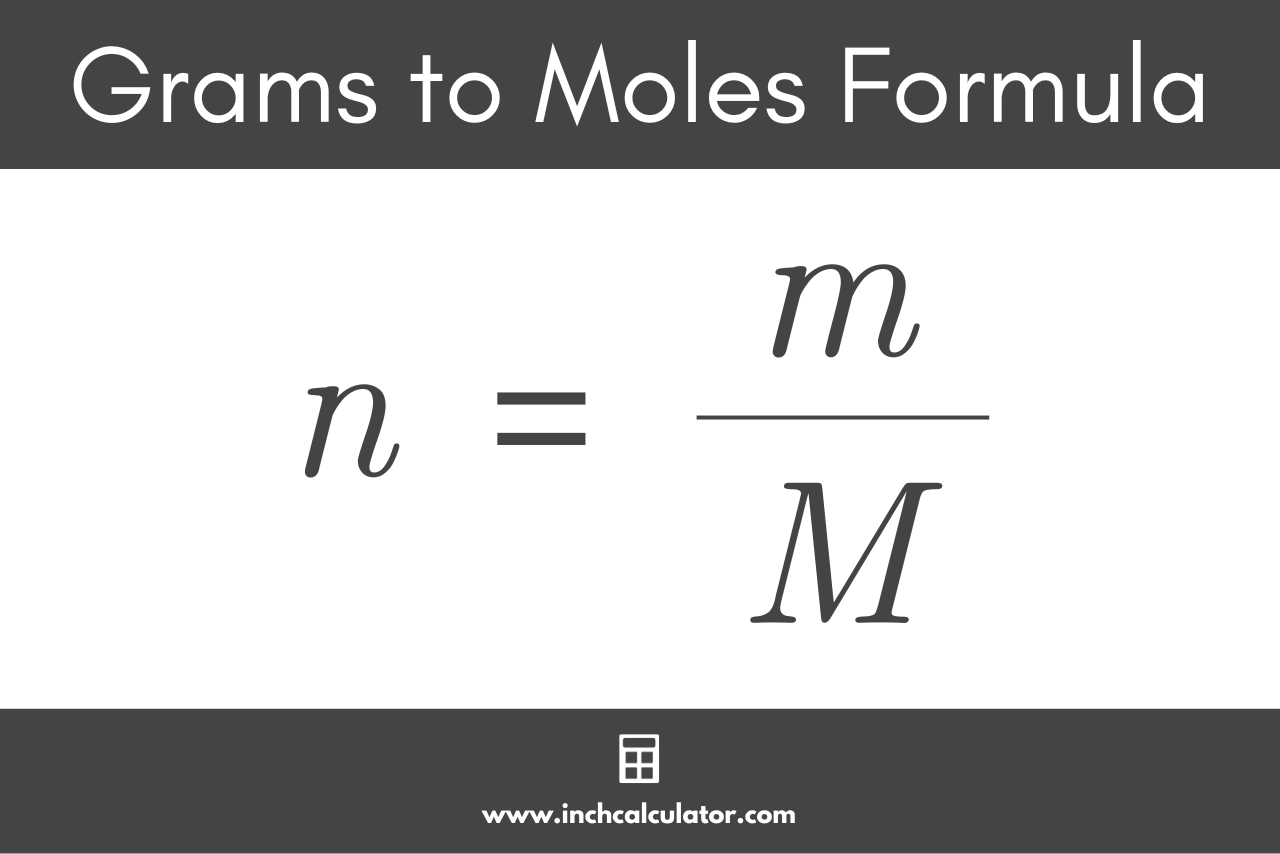


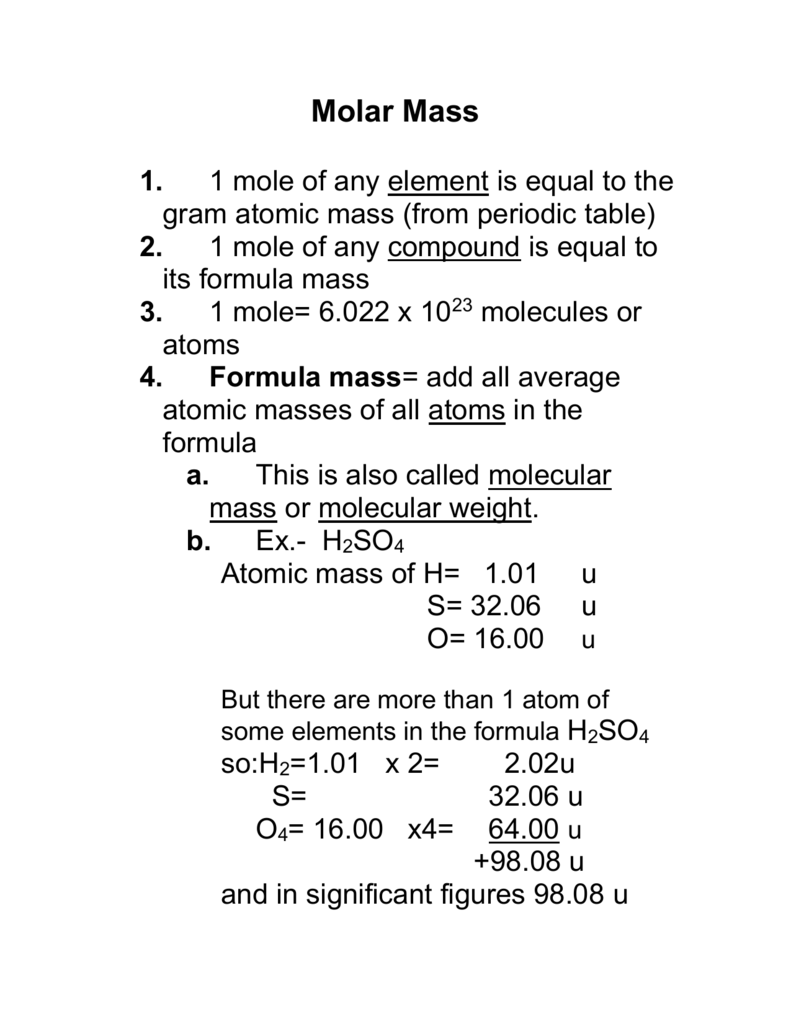

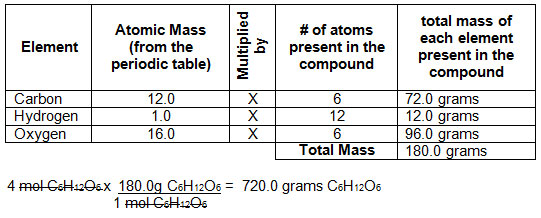
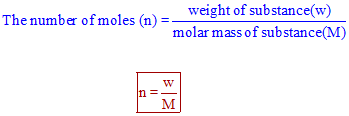
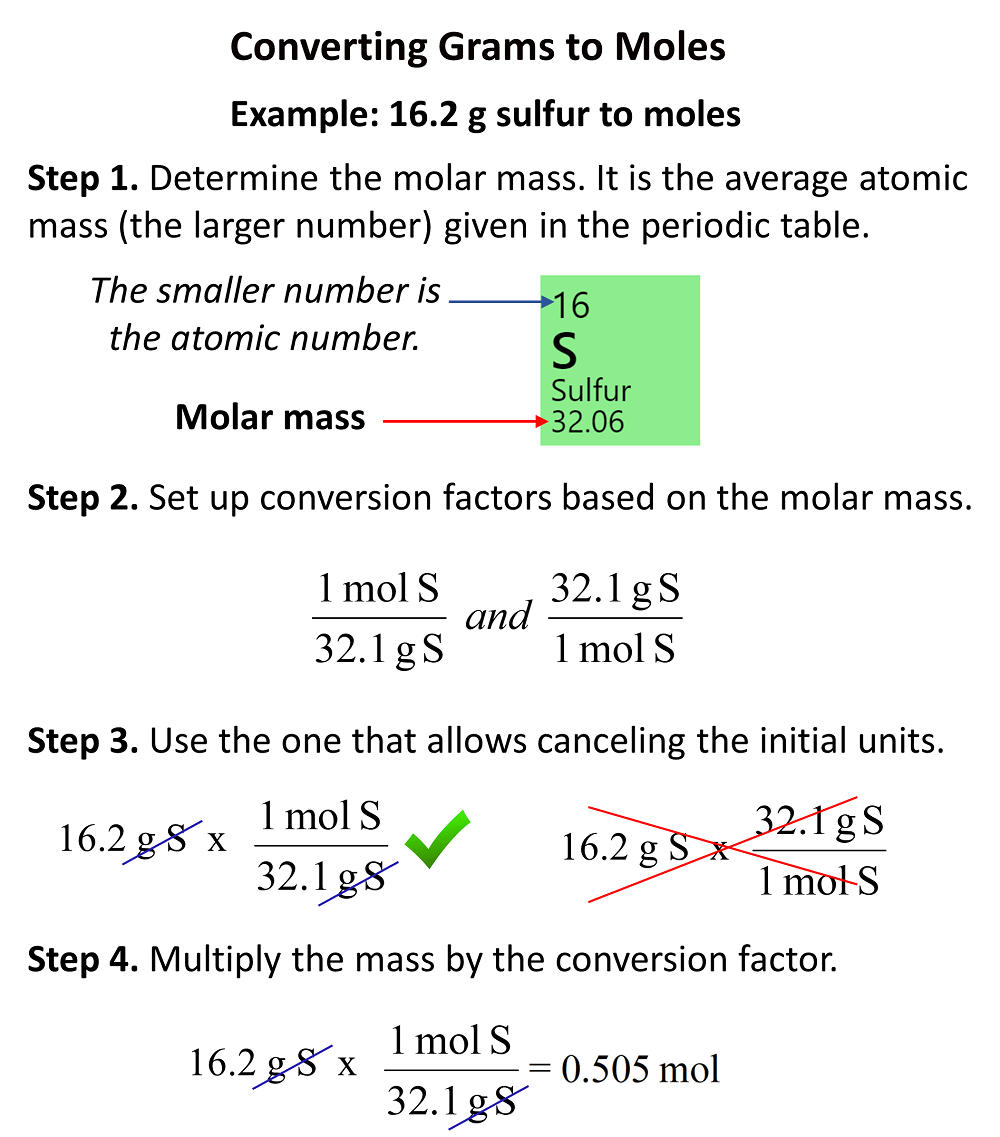
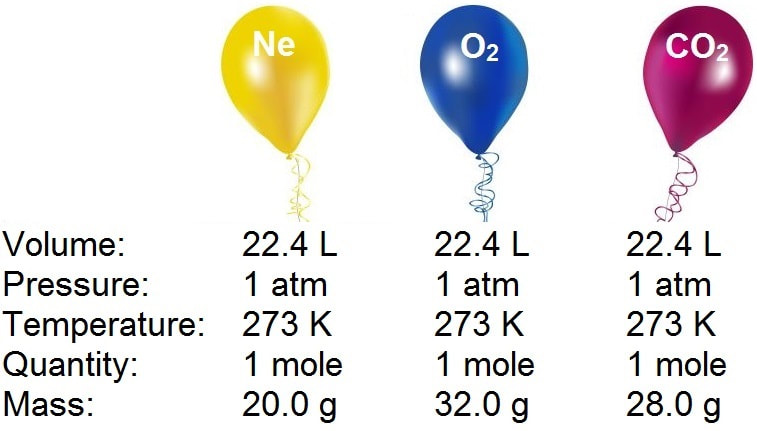
:max_bytes(150000):strip_icc()/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png)